
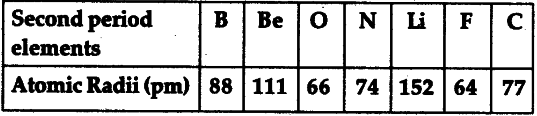
As the atomic number increases down a group, there is again an increase in the positive nuclear charge. The atomic radius of atoms generally increases from top to bottom within a group. The effect lessens as one moves further to the right in a period because of electron-electron repulsions that would otherwise cause the atom’s size to increase. Since the force of attraction between nuclei and electrons increases, the size of the atoms decreases. These electrons are gradually pulled closer to the nucleus because of its increased positive charge. Within a period, protons are added to the nucleus as electrons are being added to the same principal energy level. There are some small exceptions, such as the oxygen radius being slightly greater than the nitrogen radius. The atomic radius of atoms generally decreases from left to right across a period. The atomic radius is defined as one-half the distance between the nuclei of identical atoms that are bonded together.įigure 2. Atomic radii of the representative elements measured in picometers. In order to standardize the measurement of atomic radii, the distance between the nuclei of two identical atoms bonded together is measured. However, orbital boundaries are fuzzy and in fact are variable under different conditions. The size of an atom is defined by the edge of its orbital. This data helps us understand why some molecules fit together and why other molecules have parts that get too crowded under certain conditions. One of the ways we can express the size of atoms is with the atomic radius. The size of atoms is important when trying to explain the behavior of atoms or compounds. Knowing the sizes of objects we are dealing with can be important in deciding how much space is needed. We can get more squirrels into that same space than we can people for the same reason. We can get more people into a given space than we can elephants, because the elephants are larger than people. The crowd capacity depends on the amount of space in the venue, and the amount of space depends on the size of the objects filling it.
Even an outdoor event can fill up so that there is no room for more people. How can all of these people fit in such a small space?Įvents draw large numbers of people to them.


 0 kommentar(er)
0 kommentar(er)
